The Syner-KINASE® solution: thrombolytic therapy for catheter occlusions
The Syner-KINASE® solution: thrombolytic therapy for catheter occlusions
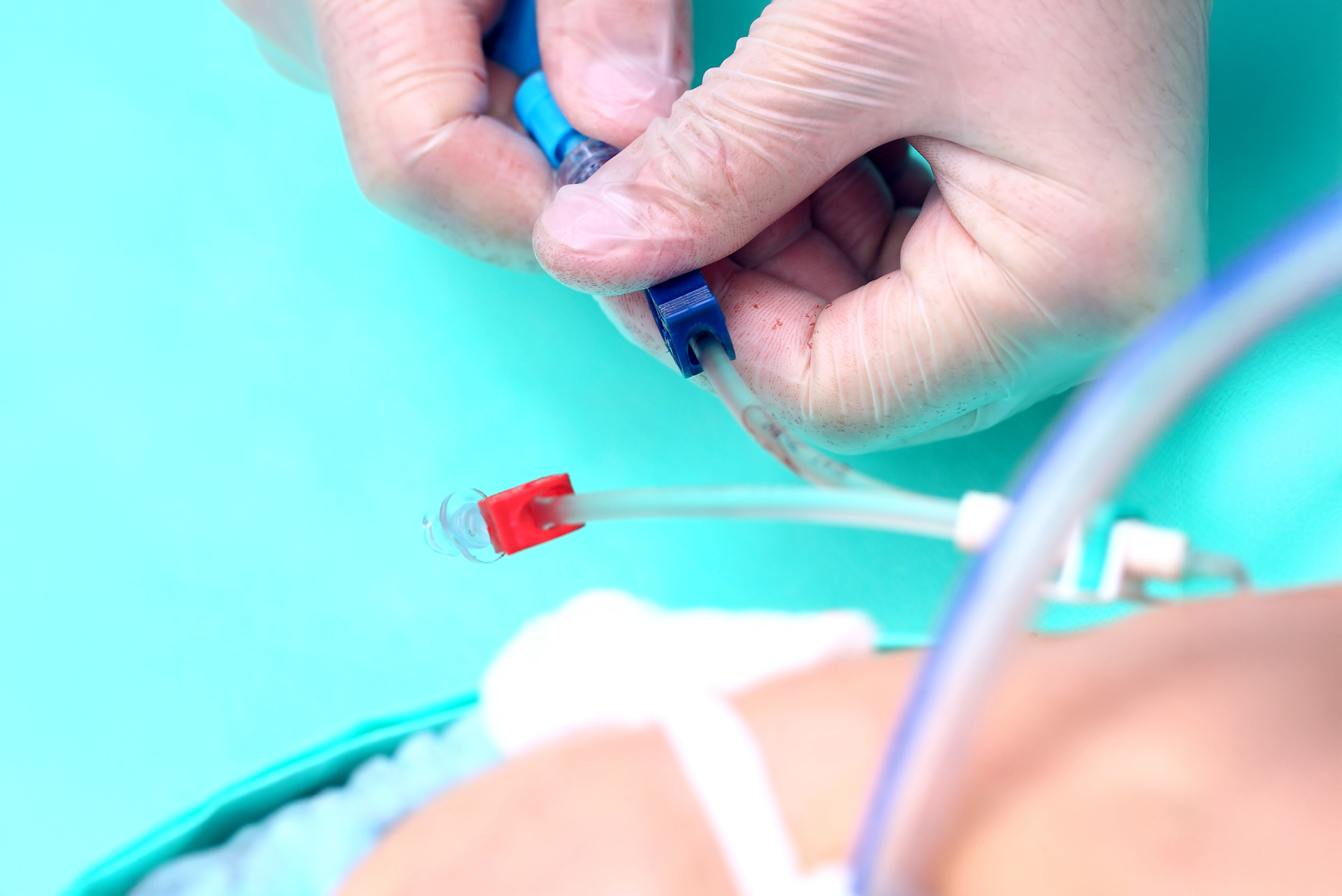
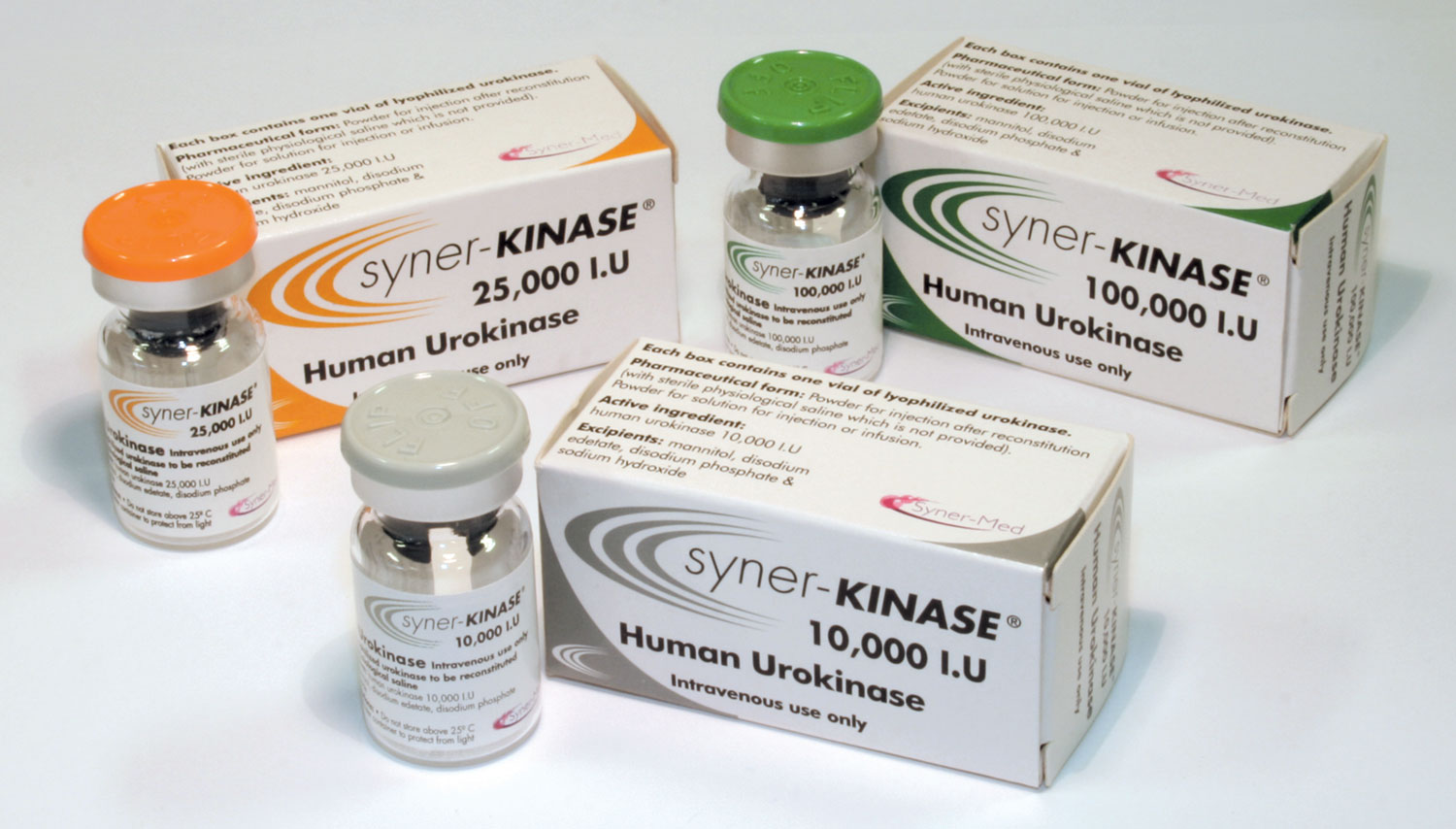
Access to a reliable and efficient thrombolytic agent is essential in settings where a blocked catheter can cause significant treatment delays. Syner-KINASE (urokinase) can provide a versatile and clinically proven solution that can help to restore catheter function quickly, supporting seamless patient care.

How Syner-KINASE works as a plasminogen activator
How Syner-KINASE works as a plasminogen activator
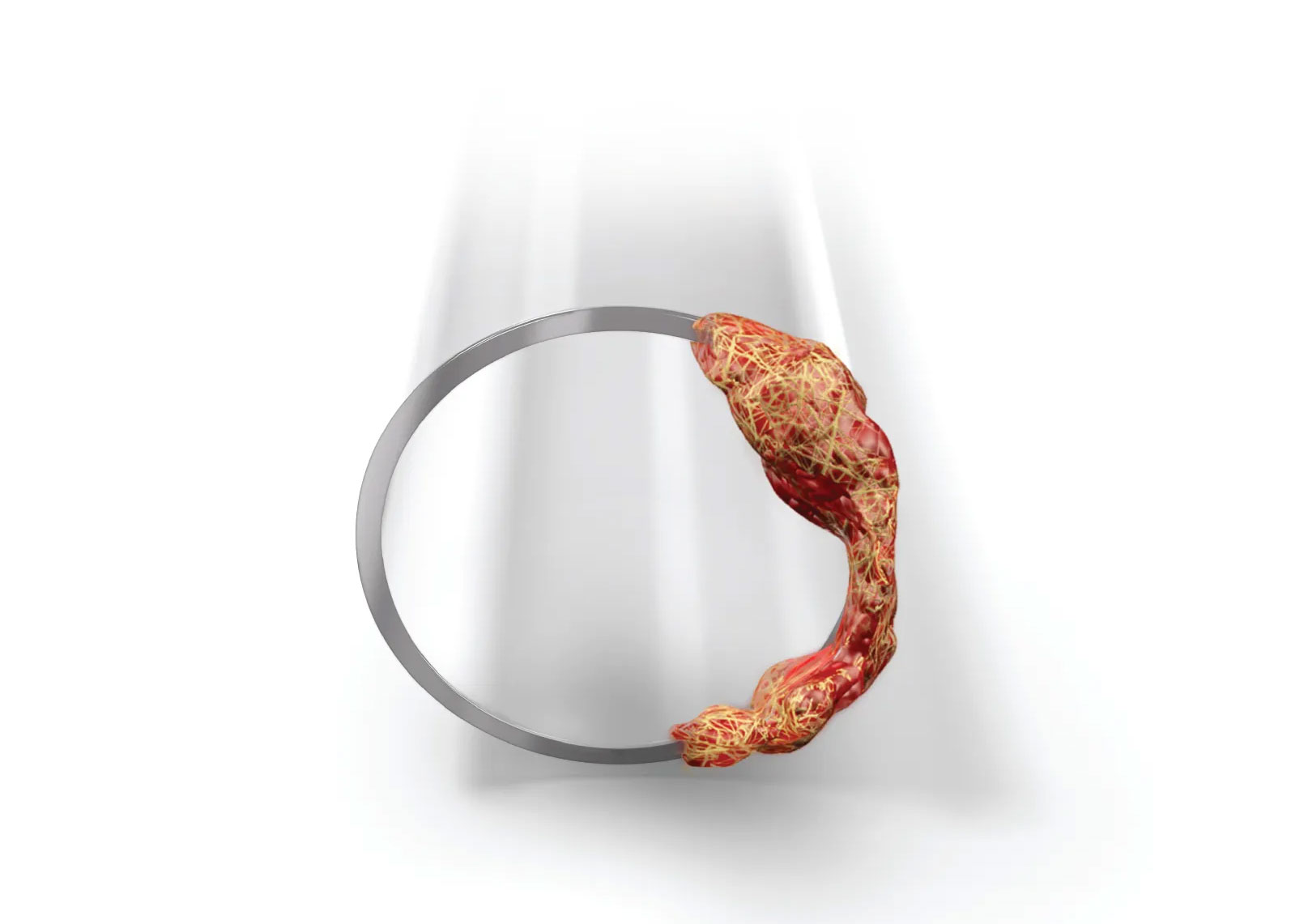
Syner-KINASE converts plasminogen – a protein abundant in the thrombus – into plasmin, an enzyme that breaks down the fibrin structural component of blood clots. By targeting the fibrin network directly, this thrombolytic solution aids rapid clot dissolution and efficient catheter clearance.
Contraindications, warnings and adverse effects
As a thrombolytic, Syner-KINASE is contraindicated in patients at serious risk from bleeding. It should be used with caution in patients where the risk may be increased, and its use should be weighed against the anticipated benefits of treatment.
See the full Summary of Product Characteristics (SmPC) here: https://www.medicines.org.uk/emc/product/7565
Contraindications
• Hypersensitivity to the active substance or excipients
• Active, clinically relevant bleeding
• Recent severe gastrointestinal bleeding, major surgery, cerebrovascular accident or trauma, or obstetric delivery
• Severe hypertension or severe hepatic/renal insufficiency (unless on renal replacement therapy)
• Coagulation defects and severe thrombocytopenia
• Aneurysm or arteriovenous malformation
• Intracranial neoplasm or other neoplasm with risk of haemorrhage
• Acute pancreatitis, pericarditis, bacterial endocarditis or sepsis
Warnings and precautions
• Conditions where there is an increased risk of bleeding
• Bleeding from sites of percutaneous trauma
• Haematoma formation
• Avoid arterial invasive procedures if possible
• If bleeding occurs during treatment, Syner-KINASE should be stopped immediately
Adverse effects
Very common:
• Haemorrhage, including from puncture or wound site
• Decrease in haematocrit without clinically detectable bleeding
• Transient increase in transaminases
Common:
• Fever, chills
• Gastrointestinal, intracranial, retroperitoneal, urogenital and muscle haemorrhage
• Stroke
Uncommon:
• Intrahepatic haemorrhage
• Renal failure
Rare:
• Hypersensitivity reactions (urticaria, dyspnoea, hypotension, flushing, rash)
• Vascular pseudoaneurysm
• Macroscopic haematuria
Very rare:
• Anaphylaxis
Discover more about Syner-KINASE
Discover more about Syner-KINASE
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse events should also be reported to Syner-Med (PP) Ltd. Tel: +44 (0)208 655 6380
For Syner-KINASE prescribing information, click here