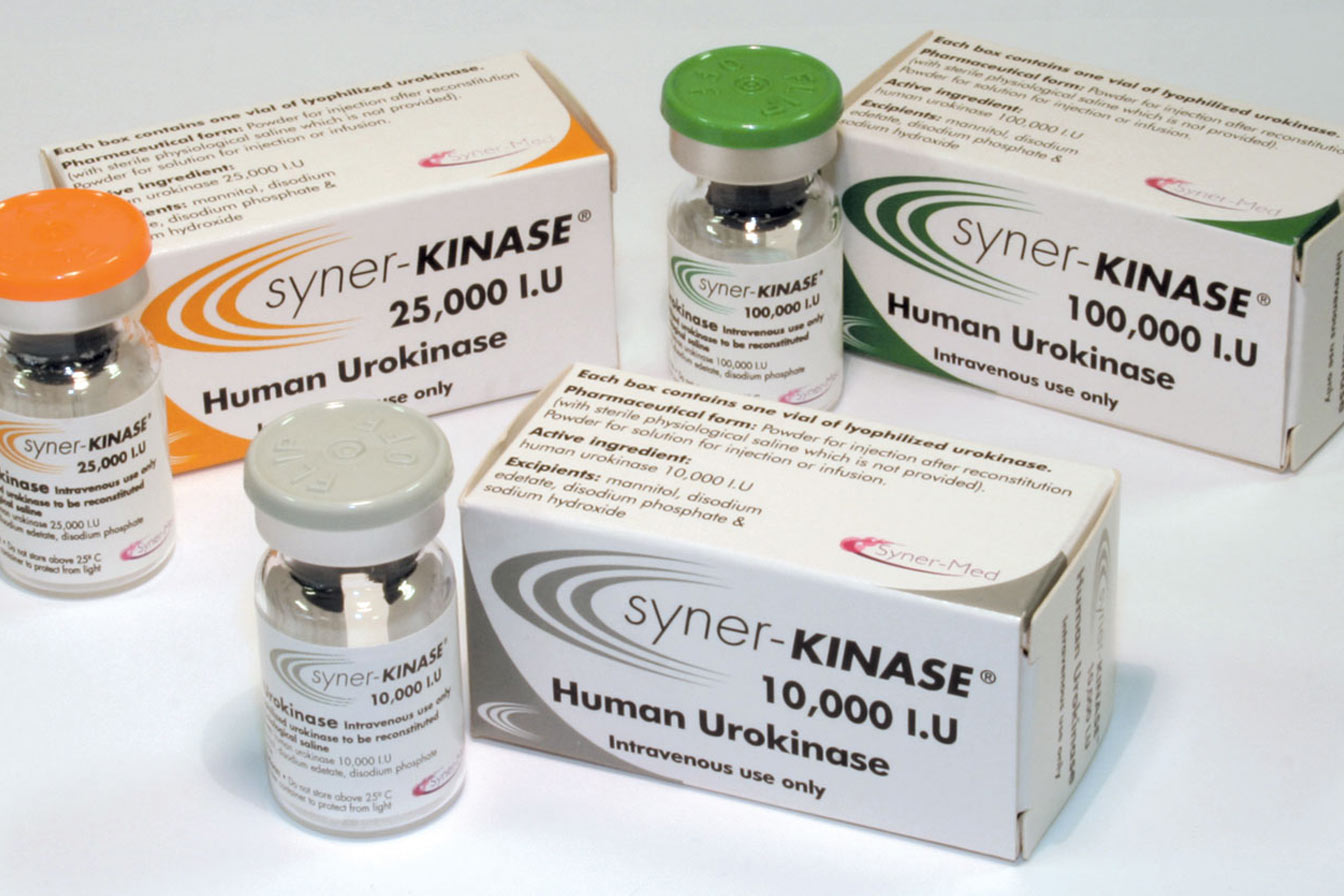
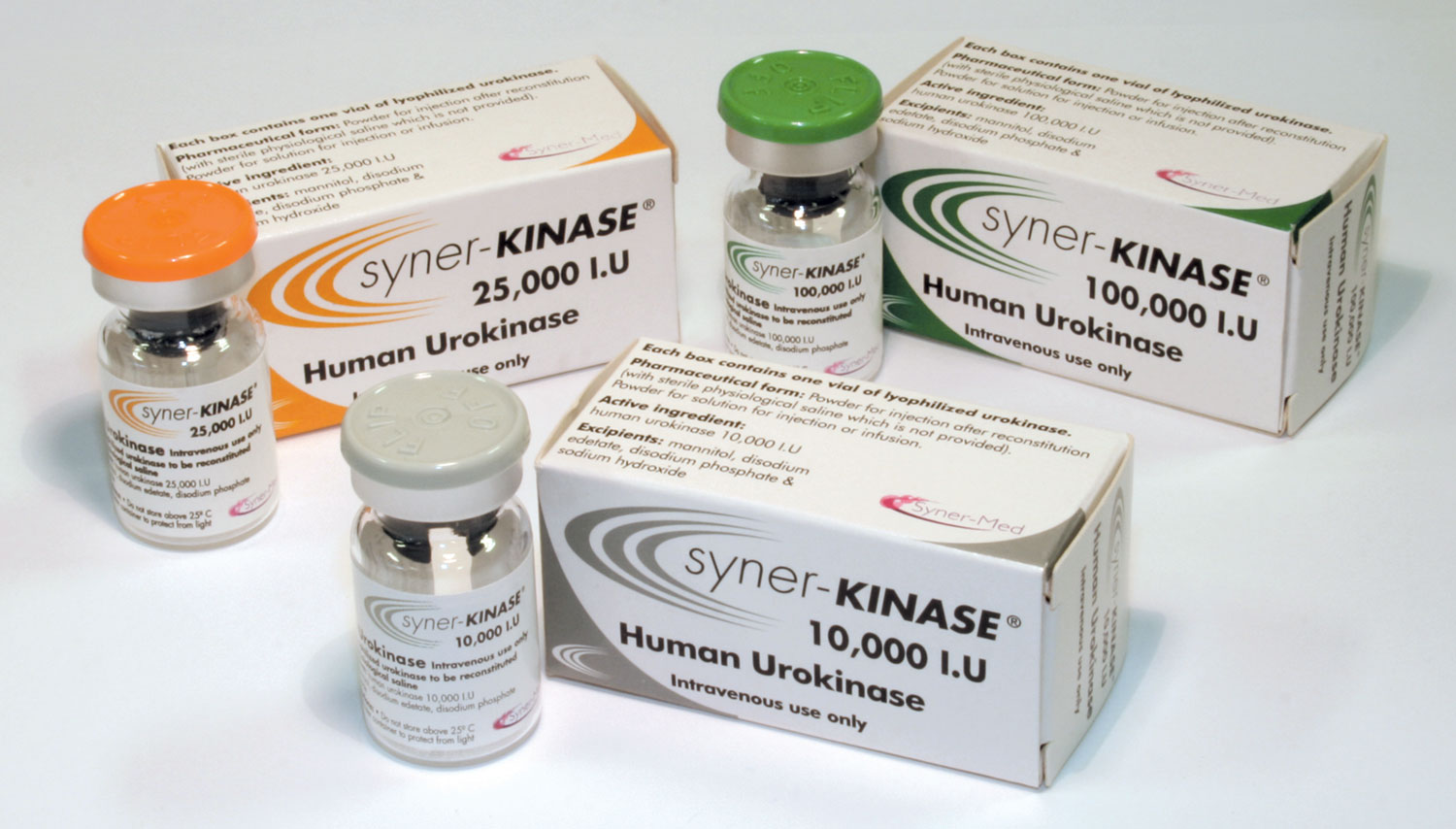
Syner-KINASE (urokinase) is a clinically proven thrombolytic agent designed to dissolve blood clots and restore catheter patency. It delivers clot dissolution through dual administration options – lock or infusion – to help minimise treatment disruptions and reduce the need for catheter replacements.
Key features and benefits
Key features and benefits
Discover more about Syner-KINASE
Discover more about Syner-KINASE
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse events should also be reported to Syner-Med (PP) Ltd. Tel: +44 (0)208 655 6380
For Syner-KINASE prescribing information, click here
References
- Syner-Med. Data on File. Resource Impact Model Evaluating Syner-Kinase® for Catheter Occlusion Management in NHS Settings. Model Version 1.0.; 2025.
- Syner-Medica Ltd. 2006. Syner-KINASE 100,000 IU powder for solution for injection/infusion – summary of product characteristics (SmPC). https://www.medicines.org.uk/emc/product/7565/smpc/print